OICR-supported trial finds new, more sensitive imaging technique can inform treatment decisions and benefit those with recurring prostate cancer
Prostate cancer is the most common type of cancer found in men, but managing the disease is difficult because not all prostate cancers are aggressive and overtreatment can lead to unnecessary side effects, such as hormone imbalances, bowel function issues and erectile dysfunction. After initial treatment, prostate cancer patients are often monitored with a prostate specific antigen (PSA) blood test, but this test provides no information about the location and the extent of the disease. Even with traditional bone scans and CT scans, remnant traces of the disease are difficult to find and often go undetected.

A few years ago, a new, more sensitive type of imaging technique had shown promise in early clinical studies abroad and Dr. Glenn Bauman, Radiation Oncologist at the London Health Sciences Centre, wanted to bring this technique into his practice. He recognized the potential benefits of this method, but didn’t realize how much it could impact the lives of his patients.
Bringing advances to local patients
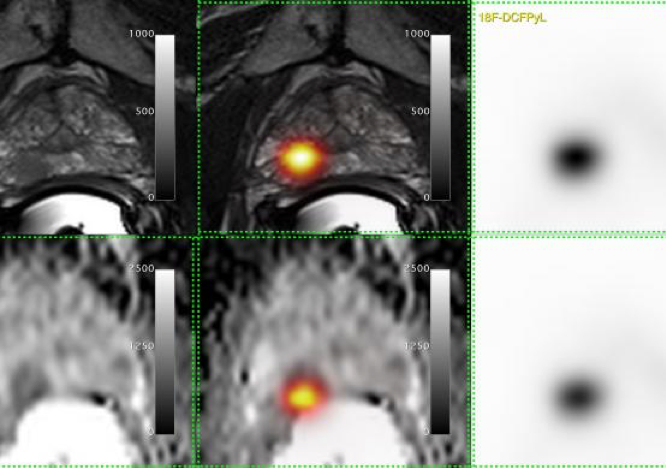
The new technique, which was originally developed at the John Hopkins Hospital in Baltimore, consisted of a chemical probe, called [18-F]-DCFPyL, which would attach only to prostate cancer cells and light up in positron emission tomography (PET) scans. It can detect very small traces of a tumour that has returned after treatment or spread to a different part of the body.
Bauman teamed up with the co-inventor of [18-F]-DCFPyL, Dr. Martin Pomper, and the Centre for Probe Development and Commercialization (CPDC) to bring this probe to patients in Ontario. CPDC implemented the stringent manufacturing processes needed to create this probe and in March of 2016, Lawson’s researchers were the first to use this technique to scan a patient at St. Joseph’s Hospital in London.
“We teamed up with experts in [18-F]-DCFPyL from the U.S. and experts in prostate PET/CT from Australia to adopt this new technique, benchmark our methods and learn from their experience,” says Bauman. “It’s with collaborations like these that we can accelerate the implementation of new methods to help patients in Ontario.”
Evaluating the benefits for those with prostate cancer
Clinical studies are needed to evaluate the effectiveness new medical techniques in practice. For this technique, Bauman and collaborators needed to test whether it’s improved accuracy and sensitivity could help make better treatment decisions.
“Treatment plans for prostate cancer differ depending on the cancer’s size and location. Whether a cancer returns in the prostate, the pelvic area or elsewhere makes a big difference,” says Bauman. “We needed to test if more sensitive imaging techniques could help patients make better treatment decisions.”
Bauman led the design and development of the Advanced Prostate Imaging of Recurrent Cancer After Radiotherapy (PICs) study to evaluate [18-F]-DCFPyL PET/CT imaging. With OICR’s support over the following two years, PICs enrolled 80 men and scanned them with both traditional imaging methods and with [18-F]-DCFPyL PET/CT.
The study group found that not only can [18-F]-DCFPyL PET/CT detect smaller traces of the disease earlier when it is more manageable, this technique changed treatment recommendations for two in every five patients.
“With this technique, we were able to clarify and reclassify a lot of the traditional scans that were previously uncertain,” says Bauman. “This means that we were able to give prostate-directed treatment with confidence for patients whose cancers reemerged in their prostate and avoid the negative side effects of systemic hormone therapy for these patients.”
Bauman says that the technique also detected double the number of cancers outside of the prostate which were too small to be detected using traditional imaging alone.
Translating clinical findings into practice
Just three years after the first [18-F]-DCFPyL PET/CT scan was taken in Canada, Bauman has embarked on the next stage in translating these findings into routine practice. He and collaborators have teamed up with Cancer Care Ontario to provide access to the [18-F]-DCFPyL PET/CT technique in Toronto, London, Hamilton, Ottawa and Thunder Bay as part of a provincial registry program.
[18-F]-DCFPyL PET/CT can be applied to other challenges that patients and clinicans face with managing prostate cancer, including monitoring how patients respond to treatments. Notably, investigators in Hamilton are investigating how these scans can help predict a patient’s response to treatment in the OICR-supported MISTR trial.
“We have been sufficiently encouraged by our results from the PICs study, through which we have demonstrated the value of this intervention and how it can benefit men with prostate cancer,” says Bauman. “I’m proud to help bring better technologies to our patients in need and enable the adoption of these technologies throughout the province.”