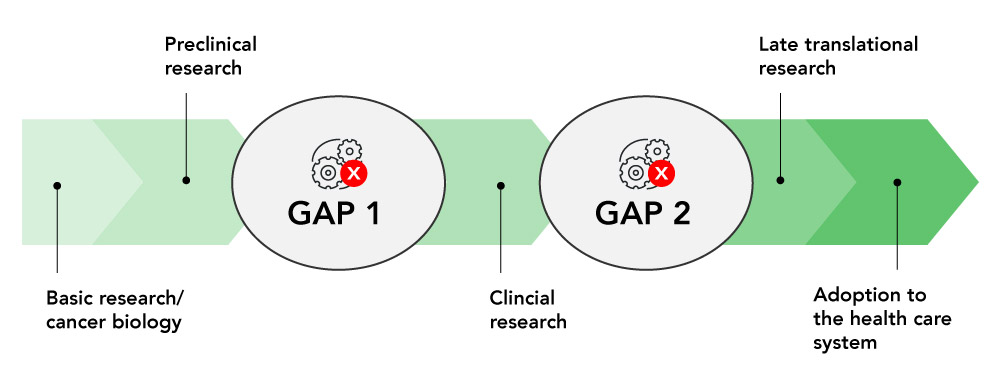
Implementation science allows OICR to bridge the gaps between research and its use in real-world settings, especially clinical medicine.
New discoveries by Ontario’s world-leading cancer researchers have the potential to transform how patients are diagnosed and treated. Yet less than half of all clinical innovations are adopted into clinical practice, and those that are adopted can take years to reach patients
We help establish pathways to assess new tools and technologies in cancer prevention, diagnosis and therapy, helping more innovations get tested and implemented in the Ontario healthcare system. With a focus on clinical impact, we support the evaluation of new tests, engage with policymakers and healthcare providers to promote innovation and study strategies to advance the uptake of evidence-based solutions.

Victor Szymanski
Project Manager, Implementation Science
vszymanski@oicr.on.ca
Translational cancer research must help deliver innovations that work to the people who need them.
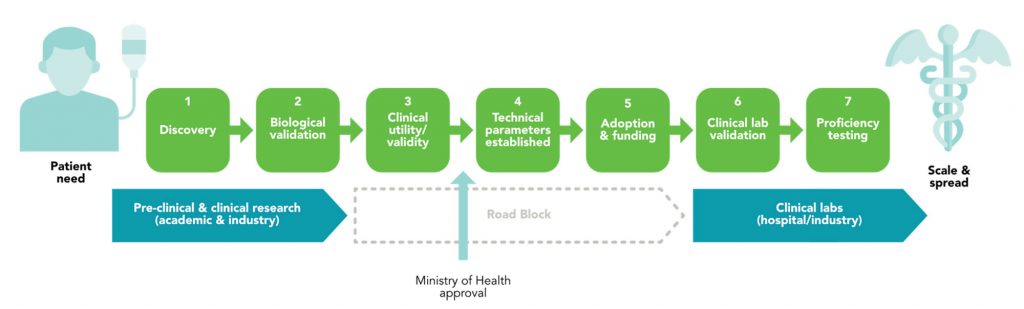
Using Implementation Science (IS) to facilitate phases of oncology biomarker development and adoption. Oncology biomarker development and adoption begin with identifying a specific patient need, which drives the Discovery (1) of potential biomarkers through rigorous research. These biomarkers then undergo Biological Validation (2) in pre-clinical studies and clinical research settings, often through collaborative efforts between academic researchers and industry partners.
At this stage, however, significant roadblocks often arise, particularly in the progression of research findings into clinical practice. Ensuring that the biomarkers are relevant and effective in addressing the identified patient need requires a standardized method of evaluating them in order to demonstrate Clinical Utility and Validity (3). The Establishment of Technical Parameters (4), such as sensitivity, specificity, and reproducibility of the assays used to detect them, is an important component of lab regulation and standardization, but is not currently well supported by funders. Finally, adoption of a new biomarker requires approval from the Ministry of Health. This is followed by Securing Funding for using the biomarker for patient care, including clinical validation to ensure good lab practice and widespread Adoption (5), both of which can be challenging.
We address these challenges by bridging the gap between research and practical application. By facilitating clinical lab validation and supporting proficiency testing through our Innovation Pathway and Evidence Framework, we plan to ensure Clinical Utility and Validity (3) demonstrating that we know that they work consistently, their cost-effectiveness, and the requirements for Ministry approval.
Through our Implementation Lab project, we can Establish Technical Parameters (4) by determining which assays can effectively be used to test for this biomarker, including answering questions about detection, measurement and reproducibility metrics, as well as workflow and cost for each assay investigated.
Our Implementation Lab and Innovation Pathway also facilitate Adoption & Funding (5), establishing how best practices are shared for new biomarker assays, who pays for the test, and who is eligible for it. Additionally, the establishment of the Implementation Lab would lay the groundwork to determine how we assess real-world effectiveness by facilitating an information sharing platform between clinical labs.
These projects enable the progression of biomarker testing toward Clinical Lab Validation (6) and Proficiency Testing (7), leading to scaling & spread in clinical labs through widespread adoption and ultimately improving diagnostic and therapeutic options for cancer patients through robust, validated, and standardized testing methods.
Research discoveries have led to interventions, tools and programs to better prevent, diagnose and treat cancer. In some cases, these innovations are underused or overused. Through the Innovation to Implementation (I2I) funding competition, OICR seeks to bridge the gaps between research products and their use in real-world settings by facilitating behaviour or system changes.
The goal of I2I awards is to fund research activities to study ways to speed up the appropriate adoption of innovations. OICR is committed to providing research funding to help support a sustainable health system that optimally delivers these innovations in a fair and equitable way to those who will benefit.
The concept of a systematic pathway for evaluating new molecular tests was developed with multi-sector support, as documented in the OICR position paper of 2019. It was refined and presented to stakeholders, including Ontario Health (OH), Cancer Care Ontario (CCO), and the Ministry of Health (MoH). OH and CCO agreed to adopt a systematic approach, with OH refining the necessary governance mechanisms.
The innovation pathway consists of three parts: appraisal, evaluation, and implementation. The appraisal phase assesses new discoveries for clinical utility and prioritizes them. The evaluation phase involves rigorous analysis using randomized control trials and cohort studies to validate the innovation’s effectiveness and safety. Successful innovations advance to the implementation phase, using a “scale and spread” model for rapid expansion. Streamlined decision-making and transparent governance ensure that only the most effective innovations are adopted, improving patient care and outcomes.
The Implementation Lab (IL) is a virtual platform designed to streamline the assessment of commercially available assays to measure biomarkers of interest. Currently, clinical labs independently select assays to measure biomarkers that become standard of care. This process of choosing between multiple available assays to measure a new biomarker can be costly and can be curtailed due to lack of funding, or because any given lab has access to only a subset of available platforms.
IL, therefore, uses the existing infrastructure of clinical labs, linking them through a shared virtual platform to standardize multiple commercial assays that could be used to determine if a specific biomarker or set of biomarkers for a particular disease are of interest. IL then identifies an appropriate set of samples to test the various assays selected, and also invites clinical labs across the country to engage in the assessment. Samples are shared between the participating labs through the virtual platform, and all selected assays are assessed using the same set of samples.
The final results include the performance characteristics, associated workflow and costs for each assay assessed, as well as any identified limits. This information is then made available to funders and to clinical labs who wish to see how their assay of choice performed. Labs can then make informed decisions as they choose the assay they wish to validate, and clinical validation can then proceed within each clinical lab wishing to offer testing for that biomarker or disease.By leveraging existing lab infrastructure and introducing a new data-sharing platform, the IL maximizes efficiency and collaboration.
The Evidence Framework, developed in collaboration with the Canadian Drug Agency (CDA), is an evaluation tool in the form of a checklist that helps decision-makers in healthcare choose which new oncology biomarkers should be used to help cancer patients. Right now, there’s no standard way to evaluate the clinical validity of a biomarker. This checklist is crucial because it ensures that we evaluate biomarkers based on standardized criteria.
Removing the guesswork and subjectivity from the process is crucial, and by partnering with CDA, an expert organization that specializes in evaluating healthcare technologies and making evidence-based recommendations, we work with collaborators who bring valuable experience and expertise to ensure the checklist is comprehensive and effective.
We can ensure that the checklist meets national standards and can be used consistently across the country. This collaboration ensures that decisions about new treatments are based on the best available evidence, promoting patient safety and improving outcomes.
The Center for Implementation
Located in Toronto, Dr. Julia Moore’s Center for Implementation is known for its role in advancing health research through a combination of professional development, collaborative projects, and implementation support.
The Inspiring Change 2.0 Mini course is a free and self-paced virtual training program that introduces participants to the core concepts of Implementation Science.
https://thecenterforimplementation.com/inspiring-change
To support implementation research, the Center for Implementation also provides networking opportunities, fosters a community of practice, and conducts research and evaluations. Their specialized courses and training in implementation science, available for a fee, cover essential topics like sustainability planning, systems thinking, adaptations, and context assessment. Additionally, they offer personalized consultation and practical tools.
https://thecenterforimplementation.com/
NCI’s Implementation Science Program within the Division of Cancer Control and Population Sciences
A resource for sample grant applications and training, the Division of Cancer Control and Population Sciences of the National Cancer Institute, part of the National Institutes of Health, provides a comprehensive webinar archive and other dissemination and implementation (D&I) resources.
https://cancercontrol.cancer.gov/is/training-events/webinars
Available for open access are eight comprehensive modules from the Training Institute for Dissemination and Implementation Research in Cancer (TIDIRC). These modules encompass a wide range of topics, including introduction to implementation science, implementation theories, models, frameworks, measures, study designs, and more.
https://cancercontrol.cancer.gov/is/training-education/training-in-cancer/TIDIRC-open-access
Dissemination-Implementation.org
Dissemination-Implementation.org assists researchers and practitioners in adapting selected implementation models to their project cycle. The platform helps identify suitable measurement instruments for the model constructs, as well as appropriate implementation models for their specific research questions or practice problems.
https://dissemination-implementation.org/tool/
iPRISM and RE-AIM Guidebook for Planning, Implementation, and Sustainment
This guide by the Colorado Implementation Science Center in Cancer Control amalgamates the Iterative Practical, Robust Implementation and Sustainability Model (iPRISM) and Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) frameworks to the specific needs of cancer research projects, with the goal of being a “one-stop-shop.” By providing essential materials and clear instructions in various cancer research contexts, it supports the implementation of these frameworks during phases of planning, implementation, and sustainment. Users can embed evaluation principles within their implementation plans to assist in challenges and progress related to end-user feedback.
Fillable & Expanded CONSORT Figure PDF for Clinical Trials
The Expanded Consolidated Standards of Reporting Trials (CONSORT) figure assists in implementation in clinical trials by helping researchers determine the representativeness, generalizability, and sustainability of outcomes research. It integrates additional data regarding participation and representativeness of settings and staff, along with the sustainability of interventions after project support concludes. The Expanded CONSORT aims to provide a more concise and transparent summary of external validity and contextual factors.
Implementation Science Workshop
Watch a recording of our recent Implementation Science (IS) Workshop webinar with guest speaker Dr. Robin Urquhart, where we explored the practical applications of IS in advancing research integration into practice.
Position paper
OPINION: A pathway to faster access to life-saving cancer innovations
Implementation Science Co-Lead
Dr. Christine Williams, PhD
Executive Vice President, OICR
Implementation Science Co-Lead
Dr. Harriet Feilotter, PhD, FCCMG, FACMG
Director, Ontario Molecular Pathology Research Network
Project Manager
Victor Szymanski
Implementation Science
- Dr. David Berman of Queen’s University created a system to objectively grade bladder cancer so that it can be treated more effectively. ‘High-grade’ and ‘low-grade’ bladder cancers are treated very differently, but the process to determine grading is highly subjective. Expert pathologists will disagree on cancer grades in 20 to 40 per cent of cases. With I2I funding, Berman and colleagues will test a computer-aided grading system for bladder cancer based on quantitative measures and work with industry, academic and patient partners to help it make it available to clinicians.
- Dr. Yvonne Bombard of the University of Toronto and St. Michael’s Hospital, a site of Unity Health Toronto, is investigating how to make genetic services for cancer more equitable for all people across Ontario. Genetic testing can help identify people at high risk of developing cancer so they can be screened earlier and treated more effectively should cancer develop. But racialized communities have a more difficult time accessing genetic services and getting a definitive diagnosis, in part because most genetic research is done on people of European ancestry but also because of the barriers built into our healthcare system. With funding from OICR, Bombard and colleagues will interview people from diverse backgrounds as well as genomics researchers, healthcare providers and other stakeholders to identify barriers to accessing genetic services and work together with racialized communities on strategies to overcome them.
- Dr. Kelvin Chan of Sunnybrook Research Institute is studying the impact of CAR-T cell therapy on quality of life for patients with lymphoma. CAR-T cell therapy shows a lot of promise in treating blood cancers like lymphoma and works by training the body’s immune cells to find and kill cancer. But it is very costly to manufacture and administer, making it difficult for regulators to measure its cost-effectiveness and manage access. In their I2I study, Chan and colleagues will ask lymphoma patients about their quality of life before and after undergoing CAR-T cell therapy so that their experiences can be factored into policy decisions surrounding this potentially life-changing new treatment.
- Dr. Geoffrey Fong of the University of Waterloo is studying the positive impact of government policies aimed at reducing the negative effects of tobacco smoking, the world’s largest cause of preventable cancer. With this funding, he will work with Ontario and international experts to model the impact of ‘endgame’ tobacco control policies, like reducing nicotine levels in cigarettes to non-addictive levels, if they were to be implemented in Canada.
- Dr. Harriet Feilotter of Queen’s University and colleagues have created the Implementation Laboratory (IL), a central lab that can advise Ontario hospitals on the best way to test tumours for cancer biomarkers. I2IS funding will help them generate more evidence for the use of the lab and design educational and clinical tools to help Ontario hospitals access it.
- Dr. Monika Krzyzanowska of the Princess Margaret Cancer Centre at the University Health Network is exploring remote interventions to help cancer patients to manage the side effects of chemotherapy between visits to the clinic. Support from OICR will help Krzyzanowska and her team capitalize on the recent growth of virtual healthcare by implementing and evaluating a telephone-based symptom management program at a large Ontario cancer centre focusing on high-risk patients undergoing chemotherapy.
- Dr. Alexander Louie and Dr. Ambika Parmar of Sunnybrook Research Institute are investigating how to best determine the cost-effectiveness of publicly funding new cancer drugs for metastatic lung cancer. With OICR’s support, they and their colleagues will develop a framework to assess cost-effectiveness that considers the many sub-types of lung cancer and use the results to engage with Canadian drug-funding agencies.
- Dr. Anthony Nichols of Lawson Health Research Institute and London Health Sciences Centre is developing a molecular test to predict how patients with HPV-related head and neck cancers, the fastest rising cancers in North America, will respond to treatment. Standard treatment for these cancers is high doses of chemotherapy and radiation, which carry significant side effects, so it’s important to know which patients will benefit from treatment and which patients could be spared unnecessary side effects. Nichols and colleagues will use their I2I award to confirm that their test is accurate and explore how it could be integrated into clinical care.
- Dr. Rola Saleeb of St. Michael’s Hospital, a site of Unity Health Toronto, is exploring the use of a new tool to standardize genetic testing for glioma, the most common type of brain cancer. This technology uses a more affordable DNA sequencing technology and could make genetic testing more accessible. Existing genetic tests are done using large, expensive equipment that isn’t available in all laboratories, meaning tests are often sent away to other labs and patients must wait weeks for results before they can begin treatment. OICR funding will allow Saleeb and colleagues to develop a single test that looks for all relevant glioma biomarkers and runs on the nanopore sequencing platform, which costs only about $1,000 and can fit on a desktop.
- Dr. Kednapa Thavorn of The Ottawa Hospital and the University of Ottawa is exploring how patient voices can improve economic evaluation – an important part of how health systems assess the value of new technologies. In an earlier OICR-funded study, Thavorn and colleagues invited patients and caregivers to provide input on the financial benefits and burdens of CAR T-cell therapy, a groundbreaking immunotherapy that has delivered promising results for people with blood cancers. This new I2I study will engage a broader range of stakeholders, including patients, researchers and policymakers, to identify barriers that prevent patient engagement in economic evaluations and recommend ways to involve patients more meaningfully.
- Dr. William Wai Lun Wong of the University of Waterloo is studying real-world evidence – data generated outside of clinical trials – about the clinical impact and cost-effectiveness of CAR T-cell therapy, a promising but expensive immunotherapy. With I2IS funding, he will engage patients, healthcare providers and other stakeholders to understand how this evidence can be harnessed to guide healthcare decision-making about the future of CAR T-cell therapy.



