Receive the latest news, event invites, funding opportunities and more from the Ontario Institute for Cancer Research.
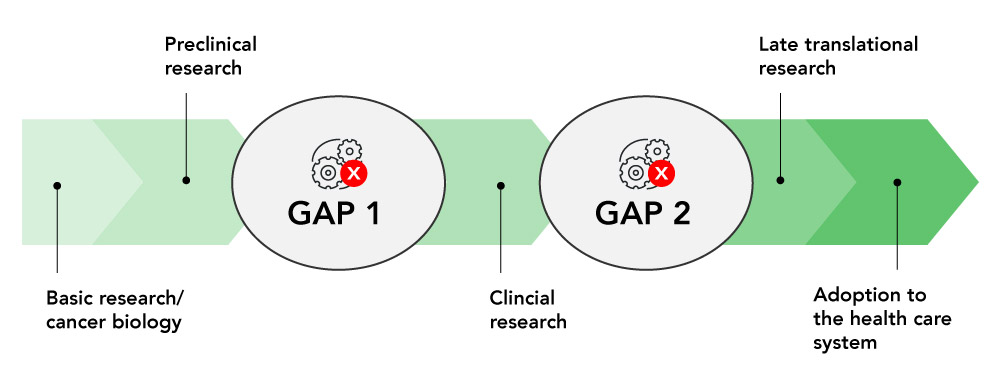
Implementation science allows OICR to bridge the gaps between research and its use in real-world settings, especially clinical medicine.
New discoveries by Ontario’s world-leading cancer researchers have the potential to transform how patients are diagnosed and treated. Yet less than half of all clinical innovations are adopted into clinical practice, and those that are adopted can take years to reach patients
We help establish pathways to assess new tools and technologies in cancer prevention, diagnosis and therapy, helping more innovations get tested and implemented in the Ontario healthcare system. With a focus on clinical impact, we support the evaluation of new tests, engage with policymakers and healthcare providers to promote innovation and study strategies to advance the uptake of evidence-based solutions.

Victor Szymanski
Project Manager, Implementation Science
vszymanski@oicr.on.ca
Translational cancer research must help deliver innovations that work to the people who need them.
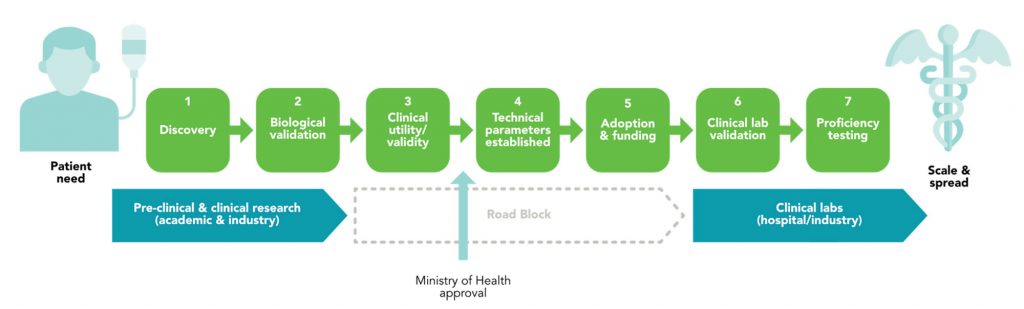
Using Implementation Science (IS) to facilitate phases of oncology biomarker development and adoption. Oncology biomarker development and adoption begin with identifying a specific patient need, which drives the Discovery (1) of potential biomarkers through rigorous research. These biomarkers then undergo Biological Validation (2) in pre-clinical studies and clinical research settings, often through collaborative efforts between academic researchers and industry partners.
At this stage, however, significant roadblocks often arise, particularly in the progression of research findings into clinical practice. Ensuring that the biomarkers are relevant and effective in addressing the identified patient need requires a standardized method of evaluating them in order to demonstrate Clinical Utility and Validity (3). The Establishment of Technical Parameters (4), such as sensitivity, specificity, and reproducibility of the assays used to detect them, is an important component of lab regulation and standardization, but is not currently well supported by funders. Finally, adoption of a new biomarker requires approval from the Ministry of Health. This is followed by Securing Funding for using the biomarker for patient care, including clinical validation to ensure good lab practice and widespread Adoption (5), both of which can be challenging.
We address these challenges by bridging the gap between research and practical application. By facilitating clinical lab validation and supporting proficiency testing through our Innovation Pathway and Evidence Framework, we plan to ensure Clinical Utility and Validity (3) demonstrating that we know that they work consistently, their cost-effectiveness, and the requirements for Ministry approval.
Through our Implementation Lab project, we can Establish Technical Parameters (4) by determining which assays can effectively be used to test for this biomarker, including answering questions about detection, measurement and reproducibility metrics, as well as workflow and cost for each assay investigated.
Our Implementation Lab and Innovation Pathway also facilitate Adoption & Funding (5), establishing how best practices are shared for new biomarker assays, who pays for the test, and who is eligible for it. Additionally, the establishment of the Implementation Lab would lay the groundwork to determine how we assess real-world effectiveness by facilitating an information sharing platform between clinical labs.
These projects enable the progression of biomarker testing toward Clinical Lab Validation (6) and Proficiency Testing (7), leading to scaling & spread in clinical labs through widespread adoption and ultimately improving diagnostic and therapeutic options for cancer patients through robust, validated, and standardized testing methods.
Research discoveries have led to interventions, tools and programs to better prevent, diagnose and treat cancer. In some cases, these innovations are underused or overused. Through the Innovation to Implementation (I2I) funding competition, OICR seeks to bridge the gaps between research products and their use in real-world settings by facilitating behaviour or system changes.
The goal of I2I awards is to fund research activities to study ways to speed up the appropriate adoption of innovations. OICR is committed to providing research funding to help support a sustainable health system that optimally delivers these innovations in a fair and equitable way to those who will benefit.
The concept of a systematic pathway for evaluating new molecular tests was developed with multi-sector support, as documented in the OICR position paper of 2019. It was refined and presented to stakeholders, including Ontario Health (OH), Cancer Care Ontario (CCO), and the Ministry of Health (MoH). OH and CCO agreed to adopt a systematic approach, with OH refining the necessary governance mechanisms.
The innovation pathway consists of three parts: appraisal, evaluation, and implementation. The appraisal phase assesses new discoveries for clinical utility and prioritizes them. The evaluation phase involves rigorous analysis using randomized control trials and cohort studies to validate the innovation’s effectiveness and safety. Successful innovations advance to the implementation phase, using a “scale and spread” model for rapid expansion. Streamlined decision-making and transparent governance ensure that only the most effective innovations are adopted, improving patient care and outcomes.
The Implementation Lab (IL) is a virtual platform designed to streamline the assessment of commercially available assays to measure biomarkers of interest. Currently, clinical labs independently select assays to measure biomarkers that become standard of care. This process of choosing between multiple available assays to measure a new biomarker can be costly and can be curtailed due to lack of funding, or because any given lab has access to only a subset of available platforms.
IL, therefore, uses the existing infrastructure of clinical labs, linking them through a shared virtual platform to standardize multiple commercial assays that could be used to determine if a specific biomarker or set of biomarkers for a particular disease are of interest. IL then identifies an appropriate set of samples to test the various assays selected, and also invites clinical labs across the country to engage in the assessment. Samples are shared between the participating labs through the virtual platform, and all selected assays are assessed using the same set of samples.
The final results include the performance characteristics, associated workflow and costs for each assay assessed, as well as any identified limits. This information is then made available to funders and to clinical labs who wish to see how their assay of choice performed. Labs can then make informed decisions as they choose the assay they wish to validate, and clinical validation can then proceed within each clinical lab wishing to offer testing for that biomarker or disease.By leveraging existing lab infrastructure and introducing a new data-sharing platform, the IL maximizes efficiency and collaboration.
The Evidence Framework, developed in collaboration with the Canadian Drug Agency (CDA), is an evaluation tool in the form of a checklist that helps decision-makers in healthcare choose which new oncology biomarkers should be used to help cancer patients. Right now, there’s no standard way to evaluate the clinical validity of a biomarker. This checklist is crucial because it ensures that we evaluate biomarkers based on standardized criteria.
Removing the guesswork and subjectivity from the process is crucial, and by partnering with CDA, an expert organization that specializes in evaluating healthcare technologies and making evidence-based recommendations, we work with collaborators who bring valuable experience and expertise to ensure the checklist is comprehensive and effective.
We can ensure that the checklist meets national standards and can be used consistently across the country. This collaboration ensures that decisions about new treatments are based on the best available evidence, promoting patient safety and improving outcomes.
The Center for Implementation
Located in Toronto, Dr. Julia Moore’s Center for Implementation is known for its role in advancing health research through a combination of professional development, collaborative projects, and implementation support.
The Inspiring Change 2.0 Mini course is a free and self-paced virtual training program that introduces participants to the core concepts of Implementation Science.
https://thecenterforimplementation.com/inspiring-change
To support implementation research, the Center for Implementation also provides networking opportunities, fosters a community of practice, and conducts research and evaluations. Their specialized courses and training in implementation science, available for a fee, cover essential topics like sustainability planning, systems thinking, adaptations, and context assessment. Additionally, they offer personalized consultation and practical tools.
https://thecenterforimplementation.com/
NCI’s Implementation Science Program within the Division of Cancer Control and Population Sciences
A resource for sample grant applications and training, the Division of Cancer Control and Population Sciences of the National Cancer Institute, part of the National Institutes of Health, provides a comprehensive webinar archive and other dissemination and implementation (D&I) resources.
https://cancercontrol.cancer.gov/is/training-events/webinars
Available for open access are eight comprehensive modules from the Training Institute for Dissemination and Implementation Research in Cancer (TIDIRC). These modules encompass a wide range of topics, including introduction to implementation science, implementation theories, models, frameworks, measures, study designs, and more.
https://cancercontrol.cancer.gov/is/training-education/training-in-cancer/TIDIRC-open-access
Dissemination-Implementation.org
Dissemination-Implementation.org assists researchers and practitioners in adapting selected implementation models to their project cycle. The platform helps identify suitable measurement instruments for the model constructs, as well as appropriate implementation models for their specific research questions or practice problems.
https://dissemination-implementation.org/tool/
iPRISM and RE-AIM Guidebook for Planning, Implementation, and Sustainment
This guide by the Colorado Implementation Science Center in Cancer Control amalgamates the Iterative Practical, Robust Implementation and Sustainability Model (iPRISM) and Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) frameworks to the specific needs of cancer research projects, with the goal of being a “one-stop-shop.” By providing essential materials and clear instructions in various cancer research contexts, it supports the implementation of these frameworks during phases of planning, implementation, and sustainment. Users can embed evaluation principles within their implementation plans to assist in challenges and progress related to end-user feedback.
Fillable & Expanded CONSORT Figure PDF for Clinical Trials
The Expanded Consolidated Standards of Reporting Trials (CONSORT) figure assists in implementation in clinical trials by helping researchers determine the representativeness, generalizability, and sustainability of outcomes research. It integrates additional data regarding participation and representativeness of settings and staff, along with the sustainability of interventions after project support concludes. The Expanded CONSORT aims to provide a more concise and transparent summary of external validity and contextual factors.
Implementation Science Workshop
Watch a recording of our recent Implementation Science (IS) Workshop webinar with guest speaker Dr. Robin Urquhart, where we explored the practical applications of IS in advancing research integration into practice.
Position paper
OPINION: A pathway to faster access to life-saving cancer innovations
Implementation Science Co-Lead
Dr. Christine Williams, PhD
Executive Vice President, OICR
Implementation Science Co-Lead
Dr. Harriet Feilotter, PhD, FCCMG, FACMG
Director, Ontario Molecular Pathology Research Network
Project Manager
Victor Szymanski
Implementation Science


