Receive the latest news, event invites, funding opportunities and more from the Ontario Institute for Cancer Research.
Cancer Therapeutics Innovation Pipeline (CTIP) supports the local translation of Ontario discoveries into therapies with the potential for improving the lives of cancer patients while creating a pipeline of promising drugs to attract partnerships and investment to Ontario.
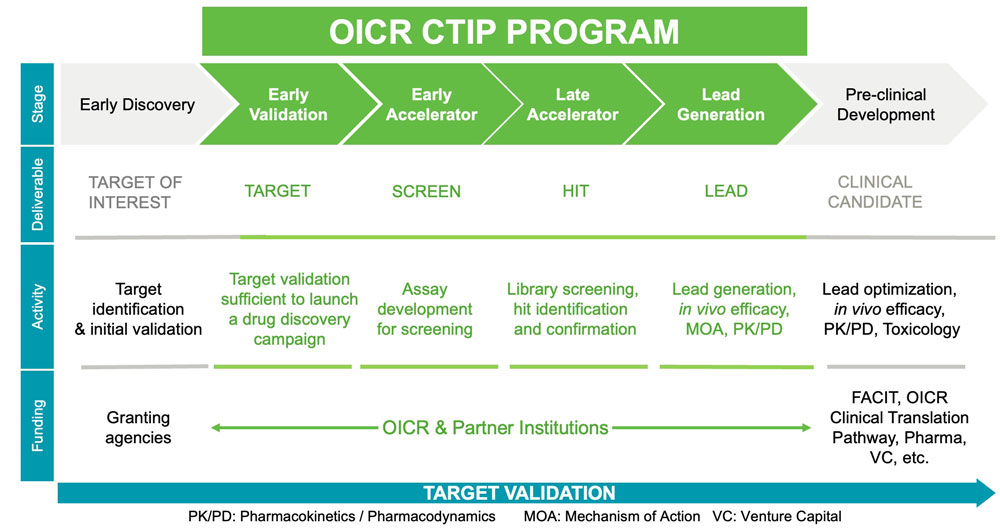
Figure 1: The Cancer Therapeutics Innovation Pipeline: Stages, deliverables, major activities, and funding sources.
CTIP funds projects in four stages of preclinical drug discovery as shown in Figure 1:















Hakim Djaballah
Founder, President and CEO, Keren Therapeutics
TPAC Chair | TPAC member since 2017

Vahe Bedian
Co-founder and Executive Advisor, Viridian Therapeutics
TPAC member from 2017 – 2021 and 2025 – Present

Elizabeth Eisenhauer
Professor Emerita, Queen’s University
TPAC member since 2017

Marc Ferrer
Director, 3-D Tissue Bioprinting Laboratory, National Center for Advancing Translational Sciences
TPAC member since 2020

George Njoroge
Chief Scientific Adviser, Kenyatta University Teaching, Referral and Research Hospital
TPAC member since 2020

Tudor Oprea
Chief Scientific Officer, Expert Systems Inc.
Professor Emeritus of Medicine, University of New Mexico Health Sciences Center
TPAC member since 2020

Ruth Plummer
Professor of Experimental Cancer Medicine
Newcastle University
TPAC member since 2025

Attila Seyhan
Director of Translational Oncology Operations, Brown University
TPAC member since 2020

Zaneta Nikolovska-Coleska
Associate Dean, Graduate & Postdoctoral Studies
University of Michigan Medical School
TPAC member since 2021