For inquiries about the WOO Network or its trials, or to discuss a potential WOO trial concept, please contact: WOONetwork@oicr.on.ca
What we do
The Window-of-Opportunity (WOO) Network is a joint effort by surgical, medical and radiation oncologists along with Ontario’s cancer research community to carry out clinical trials on patients who haven’t yet received any treatment, before they go through cancer surgery.
Why we do it
WOO trials help scientists understand if and how an emerging cancer treatment can affect tumour cells and a patient’s immune cells. By learning from each WOO trial and comparing the results across trials, our Network can help bring new treatments to patients sooner.
How we do it
WOO trials are conducted before patients receive any other therapy, including surgery. They focus on evaluating the effectiveness and mechanism of action in new therapies. Results are analyzed rigorously and compared with evidence from other WOO trials, helping advance innovative treatments so they can ultimately improve outcomes and quality of life for patients.
The WOO Network was formed in 2020 under the guidance of a multidisciplinary steering committee. Prioritized WOO studies are provided with funding and support for trial development, translational analysis and knowledge dissemination and leverage OICR’s powerful and standardized multi-omic capabilities. The WOO Network builds study capacity in Ontario through mentorship of new investigators, industry partnerships, data analytics and shared network expertise. The current focus of the WOO Network is to identify agents that rapidly modulate and enhance the immune response in cancer patients.
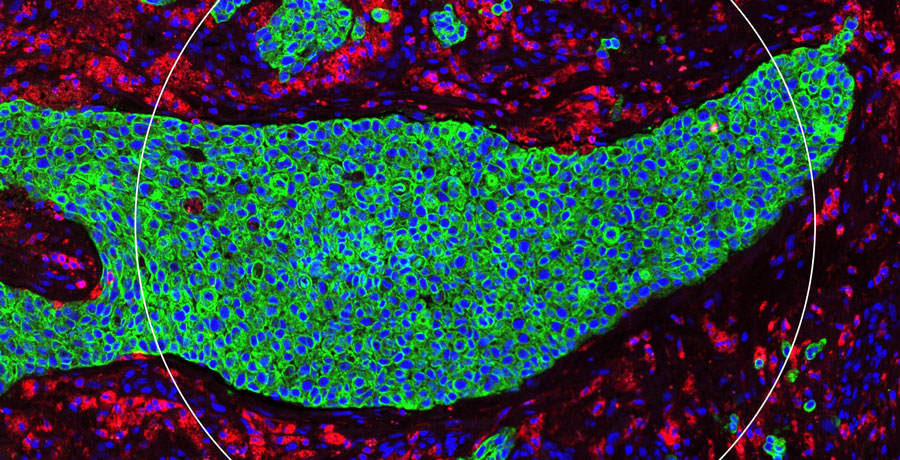
The results of WOO trials can help researchers to:
- Profile the tumour microenvironment and related systemic changes following drug exposure
- Rapidly identify the mechanism of action and on-target activity for a novel therapy
- Further precision medicine by identifying biomarkers to predict which patients will benefit from a therapeutic approach
Importance of WOO trials in cancer research:
- Inform the development of potential therapeutic approaches for neoadjuvant treatment of primary cancers
- Screen out agents with little or no activity and prioritize more promising agents for oncology drug development
- Rapidly verify drug mechanism of action, identify biomarkers for patient selection and interrogate potential resistance mechanisms
- Inform “go/no-go” decisions for new immunotherapies and their combinations

Co-Lead, WOO Network
OICR Associate

Co-Lead, WOO Network
Co-Lead, Diagnostic Development
Ontario Institute for Cancer Research
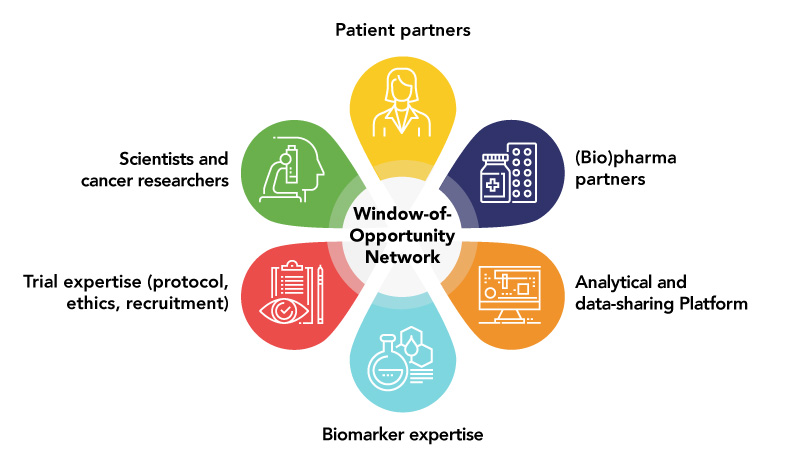
The WOO Network provides:
- Consultation with experienced WOO trialists and patient partners throughout the study process
- Guidance to promote efficient implementation, data collection, translational analysis and knowledge dissemination
- Collaboration with experts in translational science and access to leading-edge technologies, resulting in deep molecular analysis
- Funding awards for selected WOO Network trials
- Dr. Michael Ong at the The Ottawa Hospital is examining the effectiveness of pre-operative radiotherapy combined with immunotherapy in patients with bladder cancer, with a specific focus on the role of radiotherapy in ‘priming’ a patient’s immune system to enhance cancer immunotherapy.
- Dr. Erica Tsang and a team from Princess Margaret Cancer Centre, The Ottawa Hospital and Juravinski Cancer Centre are exploring the effect of combining two immunotherapy drugs (durvalumab and oleclumab) given prior to surgery, on the tumour stroma and immune function of patients with resectable pancreatic cancer.
- Dr. Dan Breadner of the Baker Centre for Pancreatic Cancer and Lawson Health Research Institute (London Health Sciences Centre) and the Schulich School of Medicine & Dentistry (Western University) is examining if stereotactic ablative radiation (SABR) therapy can reduce the size of pancreatic tumours and make surgeries more effective, while also monitoring for immune changes in and around the tumour that may increase the chance that a patient’s tumour responds to chemotherapy or immunotherapy.
- Dr. Michael Reedjik of the Princess Margaret Cancer Centre and colleagues are testing whether a drug called Anakinra, which is currently used to treat arthritis, can activate patients’ immune systems to attack triple-negative breast cancer, one of the most aggressive and deadly forms of the disease.
- Dr. Angel Arnaout and Dr. Arif Awan of the Ottawa Hospital Research Institute are leading a study investigating if combining a drug normally used for dermatological conditions (Dupilumab) with a cancer immunotherapy drug (Cemiplimab) will enhance the ability of a patient’s immune system to fight an aggressive form of estrogen-receptor positive (ER+) breast cancer.
- Dr. Sara Kuruvilla and Dr. Anthony Nichols of the London Regional Cancer Program at London Health Sciences Centre (LHSC) and Lawson Health Research Institute are investigating the effectiveness of a novel immunotherapy drug combination before surgery to remove head and neck tumours, including monitoring how patients’ immune systems respond and if they experience fewer side effects from surgery. Findings are expected to provide preliminary evidence to support further evaluation of this approach in larger clinical trials.
- Dr. Pablo Serrano and Dr. Brandon Meyers of Hamilton Health Sciences and McMaster University are studying how a common immunotherapy (Durvalumab) impacts liver tumours after patients have stereotactic body radiation therapy (SBRT) but before they have surgery, with hopes that the combination strengthens patients’ immune systems to fight cancer.
- Presurgical study in treatment-naive or early-stage recurrence patients
- Demonstrated drug safety profile
- Patient engagement is embedded in the proposal either through patient engagement at the Principal Investigator’s (PI) healthcare centre or in collaboration with OICR’s Patient and Family Advisory Council
- Highly engaged Principal Investigator, inclusion of a surgeon as lead or co-PI is strongly recommended
- Completion of patient accrual within two years
- Inclusion of trial data in WOO Network’s data platforms
- Alignment with current WOO Network research focus: Immunomodulation with emphasis on the identification of novel biomarkers of immune response
- Transforming cancer care and saving lives [Globe and Mail]